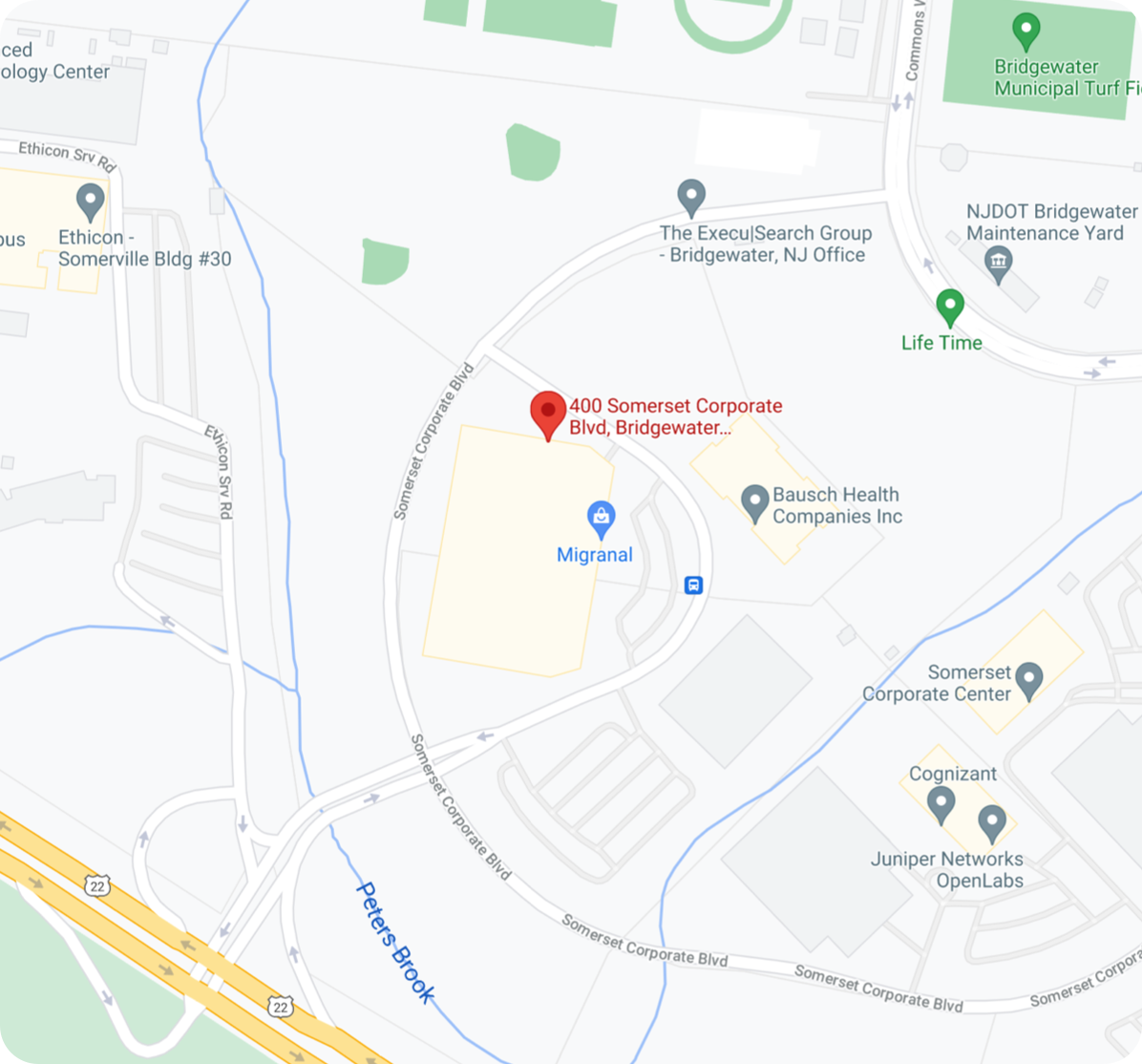
OraPharma Corporate Headquarters
400 Somerset Corporate Boulevard Bridgewater, NJ 08807, United States


We continue to make a difference in patients’ lives and to dental practices with our comprehensive suite of products to address a range of oral health concerns.
(minocycline HCl) Microspheres, 1 mg

We provide dental professionals with tools and resources to educate patients on oral healthcare.
Visit OraPharma professional education
We also collaborate with leading academic institutions and professional associations to benefit dental hygiene students and professionals. Notably, our Student Access program provides education and experience to dental hygiene students at dental hygiene schools nationwide. Since 2007, over 250 dental hygiene schools participate in this program annually.
Visit Student Access programWe are engaged in a partnership with Datum Dental Ltd. for the OSSIX product portfolio.
OSSIX portfolioWe partner with over 35 dental service organizations nationwide to support dental practices by ensuring access to our oral health products and other offerings.
PARTNER WITH USWe continually seek to collaborate and partner with academic, professional, and corporate entities to expand our offerings to help patients.
contact usRead news releases

Culture, community, inclusion, and respect shape who we are at OraPharma. We provide opportunities to support individual growth, career management, and leadership development. It is our goal to make everyone feel respected and equally appreciated so that we are all dedicated to the same cause.
400 Somerset Corporate Boulevard Bridgewater, NJ 08807, United States
1-866-273-7846 OraPharma@OraPharma.com
